Page 3 - Kidney Cancer Research Network of Canada (KCRNC) consensus statement on the role of adjuvant therapy after nephrectomy for high-risk, non-metastatic renal cell carcinoma: A comprehensive analysis of the literature and meta-analysis of randomized controlled trials
P. 3
Role of adjuvant therapy after nephrectomy for nmRCC
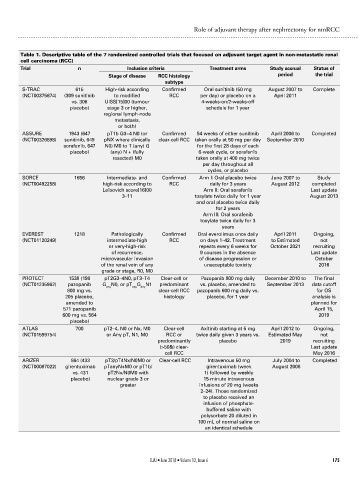
Table 1. Descriptive table of the 7 randomized controlled trials that focused on adjuvant target agent in non-metastatic renal
cell carcinoma (RCC)
Trial n Inclusion criteria Treatment arms Study accrual Status of
Stage of disease RCC histology period the trial
subtype
S-TRAC 615 High-risk according Confirmed Oral sunitinib (50 mg August 2007 to Complete
(NCT00375674) (309 sunitinib to modified RCC per day) or placebo on a April 2011
vs. 306 UISS[15]00 (tumour 4-weeks-on/2-weeks-off
placebo) stage 3 or higher, schedule for 1 year
regional lymph-node
metastasis,
or both)
ASSURE 1943 (647 pT1b G3−4 N0 (or Confirmed 54 weeks of either sunitinib April 2006 to Completed
(NCT00326898) sunitinib, 649 pNX where clinically clear-cell RCC taken orally at 50 mg per day September 2010
sorafenib, 647 N0) M0 to T (any) G for the first 28 days of each
placebo) (any) N + (fully 6-week cycle, or sorafenib
resected) M0 taken orally at 400 mg twice
per day throughout all
cycles, or placebo
SORCE 1656 Intermediate- and Confirmed Arm I: Oral placebo twice June 2007 to Study
(NCT00492258) high-risk according to RCC daily for 3 years August 2012 completed
Leibovich score[16]00 Arm II: Oral sorafenib Last update
3–11 tosylate twice daily for 1 year August 2013
and oral placebo twice daily
for 2 years
Arm III: Oral sorafenib
tosylate twice daily for 3
years
EVEREST 1218 Pathologically Confirmed Oral everolimus once daily April 2011 Ongoing,
(NCT01120249) intermediate-high RCC on days 1–42. Treatment to Estimated not
or very-high-risk repeats every 6 weeks for October 2021 recruiting
of recurrence, 9 courses in the absence Last update
microvascular invasion of disease progression or October
of the renal vein of any unacceptable toxicity 2016
grade or stage, R0, M0
PROTECT 1538 (198 pT2G3–4N0, pT3–T4 Clear-cell or Pazopanib 800 mg daily December 2010 to The final
(NCT01235962) pazopanib G N0, or pT G N1 predominant vs. placebo, amended to September 2013 data cutoff
any
any
any
800 mg vs. clear-cell RCC pazopanib 600 mg daily vs. for OS
205 placebo, histology placebo, for 1 year analysis is
amended to planned for
571 pazopanib April 15,
600 mg vs. 564 2019
placebo)
ATLAS 700 pT2–4, N0 or Nx, M0 Clear-cell Axitinib starting at 5 mg April 2012 to Ongoing,
(NCT01599754) or Any pT, N1, M0 RCC or twice daily given 3 years vs. Estimated May not
predominantly placebo 2019 recruiting
(>50&) clear- Last update
cell RCC May 2016
ARIZER 864 (433 pT3/pT4Nx/N0M0 or Clear-cell RCC Intravenous 50 mg July 2004 to Completed
(NCT00087022) girentuximab pTanyN+M0 or pT1b/ girentuximab (week August 2008
vs. 431 pT2Nx/N0M0 with 1) followed by weekly
placebo) nuclear grade 3 or 15-minute intravenous
greater infusions of 20 mg (weeks
2–24). Those randomized
to placebo received an
infusion of phosphate-
buffered saline with
polysorbate 20 diluted in
100 mL of normal saline on
an identical schedule
CUAJ • June 2018 • Volume 12, Issue 6 175